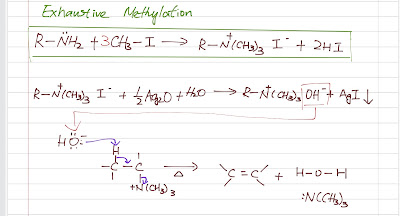
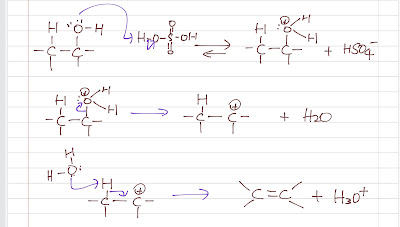
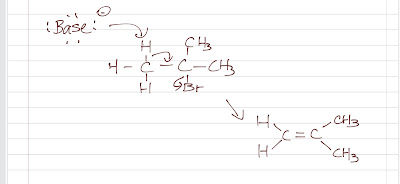
Alkene Synthesis (Part 4)
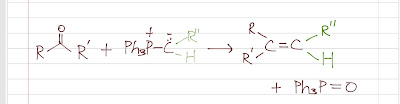
Wittig Reaction Wittig reaction turns a carbonyl ( compound that has C=O) to an alkene by reacting a carbonyl (aldehyde or ketone) with a phosphorus ylide . It is a very useful reaction that turns a C=O to a desired C=C. Making ylide Let's talk about the phosphorus ylide . It has no overall charge, but it has a negatively charged carbanion that is bonded to a positively charged phosphorus. It is prepared by a two-step reaction sequence - an SN2 in which a triphenylphosphine attacking an unhindered alkyl halide (making a positively charged phosphorus), followed by a proton abstraction by a strong base (usually butyllithium ). We know that Phosphorus and Sulfur can form more than 4 bonds using the d orbitals . One may think that the ylide should have a double bond instead of having the carbon and phosphorus ch...