Fischer Esterification of An Unknown Alcohol
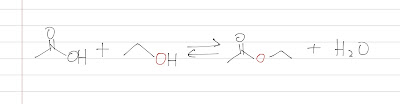
As I love jumping from here and there, let's talk about Fischer Esterification! While you may think that chemical reactions go in one way and never turn back. The truth is that a chemical reaction is always in an equilibrium! Here is the Fischer Esterification, which is always a reaction between a carboxylic acid and an alcohol. Note that the source of the oxygen of the ester group and the reaction is always in an equilibrium. Fisher Esterification Now, let's revise the concept of equilibrium and the math behind it like how to calculate the Keq. We consider concentration when we look at an equilibrium. The initial concentration is Mi for both the acetic acid and the ethanol. When the equilibrium is established, a concentration of x of ester and water is formed. In a closed environment, the product can only be formed from the reactants. So the reactants lost a concentration of x to form the product. The final concentration of the reactants is Mi-x . ...