Factors affecting rate of SN2
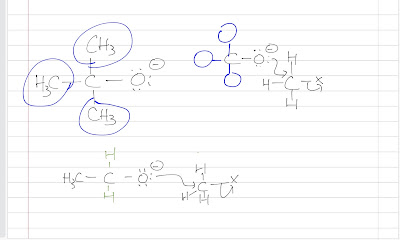
The are several factors that affect the reaction rate of SN2: Nucleophilicity (strength of nucleophile ) Substrate (the guy being attacked by the nucleophile ) while there are 2 factors affecting the nucleophilicity i . steric hindrance ii . solvent effect and 2 factors affecting the substrate i . leaving group ii . steric hindrance So actually there are 4 factors affecting the reaction rate of SN2. Let's go over it one by one. Nucleophilicity Nucleophile is a guy who loves nucleus as he has extra electrons around him. So generally, a nucleophile is negatively charged and the more negatively charge, the better nucleophile it is. We can then make a generalization that a conjugate base is a better nucleophile than its conjugate acid . For example, an ethoxide ion is more nucleophilic than its conjugate acid, ethanol. Common Pitfall: it is tempting to say that the ethoxide ion is more nucleophilic because it is more basic. W...