EAS: Halogenation
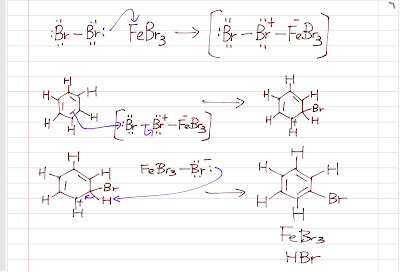
The first step of EAS is highly endothermic as the aromaticity is lost. Therefore, we need a strong electrophile to initiate the reaction. If we want to do bromination , we cannot use Br2 directly because it is not a strong electrophile (Br2 has no open octect and it is nonpolar, no formal charges). We enhance its electrophilicity by using a Br2.FeBr3 (or Br2.AlBr3 ) intermediate (FeBr3 or AlBr3 is a Lewis acid , electron acceptor, so it withdraws the electrons from Br, making it much more polar). The Fe-Br bond is more polar so that the Br is a stronger electrophile . The rest follows the general mechanism pattern. The electrophile attacks the benzene ring, forming a sigma complex. Then a proton is lost, giving off HBr and achieving aromaticity again. Chlorination is basically the same with the chlorine group instead of the bromine group. EAS: Bromination The sigma complex is stabilized by resonance: ...
