Alkene Synthesis (Part 2)
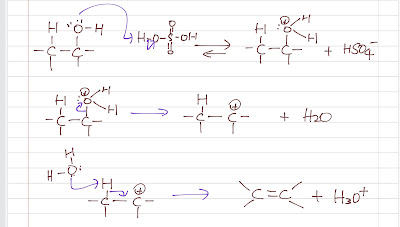
Dehydration of alcohols This is a reversible acid-catalyzed reaction. In fact, it is a common way to turn an alkene into an alcohol. To increase the yield of the product, the alkene produced is usually distilled off (an alkene has a lower boiling point than its alcohol due to the lack of hydrogen bonding) to shift the equilibrium to the product side. Concentrated sulfuric acid is used as a catalyst to protonate the -OH group to a better leaving group, H2O. Then, a E1 mechanism is followed: 1. ionization (water leaves) to a crabocation 2. a weak base (water or HSO4-) abstracts the proton to form an alkene. Dehydration of alcohol Cracking (alkane) An industrial (large scale and least expensive) way to make alkene is by the catalytic cracking of alkane (e.g. petroleum). A long chain of alkane is heated with catalyst (e.g. platinum) to form small alkenes (around 6 carbon atoms). This process of dehydrogenation is endothermic, but it has a positive entropy change...