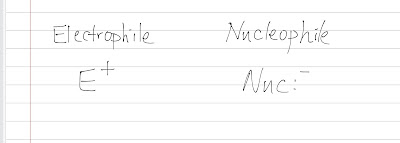SN1: Unimolecular Substitution
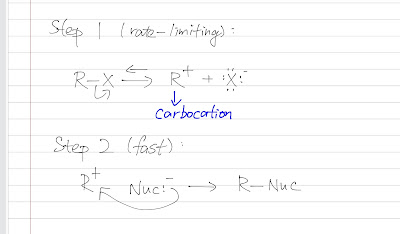
As we have already learned SN2, what would happen if the substrate is hindered and the nucleophile is a weak one? SN1 will happen. Unimolecular : SN1 is called unimolecular substitution because only one molecule (the substrate) is involved in determining the rate of reaction. This would not happen if the reaction is one step (nucleophile attacks and leaving group leaves the substrate). This reaction must involves multi steps: one is rate-liming (slow) and the other is fast (doesn't affect the rate). As it is not a concerted reaction, an intermediate is formed. Substitution : the nucelophile (from the solvent) substitutes the leaving group. SN1 is also called solvolysis because the solvent acts as the nucleophile (SN1 happens when we put a bulky substrate in a solvent). Let's look at the general mechanism: Unimolecular Substitution Step 1 is a rate-determining step which is a slow ionization that gives a carbocation intermediate. This step is slow and has a high...